Sources close to the development said the company had no further supply commitments to the WHO, and therefore, there was no suspension of any upcoming orders, reports Sohini Das.
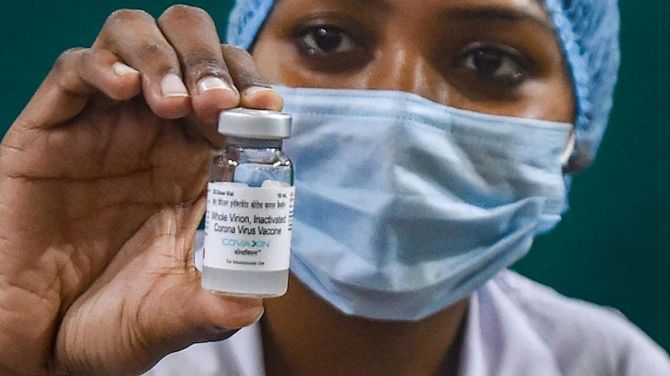
Bharat Biotech has said there were no issues with its COVID-19 vaccine or its certification, reacting after the World Health Organization suspended supplies of Covaxin through United Nations agencies.
Covaxin certificates issued remain valid, as there is no impact on efficacy and safety of the vaccine, it said.
Sources close to the development said the company had no further supply commitments to the WHO, and therefore, there was no suspension of any upcoming orders.
The WHO, however, asked Bharat Biotech to upgrade its production facilities, which the company will undertake now.
“It is like one was taking a sedan for an uphill drive, and the WHO said that having an SUV would be a better option. There are no issues on quality of the vaccine, or even the vaccine production process. Now that we are in the endemic stage, one can stop production and upgrade the facilities,” said a source without commenting on the exact nature of the observations that the global agency has made.
Hyderabad-based Bharat Biotech’s facilities were re-purposed to produce high volumes of Covaxin. These facilities were not originally built to make Covaxin in such large volumes. Therefore, upgradation is necessary, pointed out the source.
The WHO said the vaccine is effective and no safety concerns exist, but the suspension of production for export will result in the interruption of Covaxin supply.
It said the suspension is in response to the outcomes of WHO post emergency use listing inspection conducted from March 14 to 22.
During the recent WHO post EUL inspection, Bharat Biotech agreed with the WHO team on the scope of the planned improvement activities and indicated that they will be executed as soon as practical, Bharat Biotech said.
"The company was also pleased to learn from the WHO, that the necessary optimization work “Does not indicate a change in the risk-benefit ratio (for Covaxin) and the data, available to WHO, indicates the vaccine is effective and no safety concern exists”, " the company said in a statement.
This risk assessment by the WHO is based on the supply of hundreds of millions of doses of Covaxin globally.
Bharat Biotech is working to further improvements and upgrades to ensure that the production of Covaxin continues to meet ever increasing global regulatory requirements, the company said.
Bharat Biotech said on Friday that it is slowing down Covaxin production across sites as it sees decrease in demand.
The company added that it has finished supply obligations to various procurement agencies.
"For the coming period, the company will focus on pending facility maintenance, process and facility optimization activities," it said.
As all existing facilities were repurposed for the manufacture of Covaxin, with continuous production during the past year, to meet the public health emergency of COVID-19, these upgrades were due, it said.
In December, Serum Institute of India had cut down Covishield production by half after it piled up an inventory of 250 mn finished doses and 250 mn bulk doses.
More than 1 million doses of Covaxin were introduced under clinical trial mode, where safety of subjects was actively documented.
Finally, Covaxin was evaluated in around 30,000 subjects in more than 10 controlled clinical trials, resulting in more than 15 publications.
"Since patient safety is the primary consideration for any new vaccine, there can be no compromises in meeting operational excellence objectives," the company said.
Bharat Biotech is fully committed to implementing the facility improvements and upgrades to ensure that the production of Covaxin meets all global regulatory requirements, said the firm.











 © 2024 Rediff.com -
© 2024 Rediff.com -