An expert panel of India's Central Drug Authority on Friday recommended granting permission for restricted emergency use of Oxford-AstraZeneca vaccine Covishield, being manufactured by the Serum Institute of India, paving the way for the roll-out of the first COVID-19 shot in the country in the next few days.
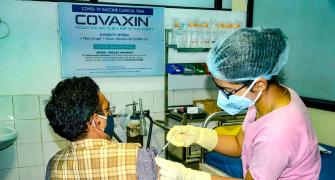
As for Bharat Biotech's Covaxin, official sources said, the Subject Expert Committee (SEC) on COVID-19 of the Central Drugs Standard Control Organisation (CDSCO) asked the firm to expedite volunteer recruitment for the ongoing phase 3 clinical trial and recommended that it may conduct interim efficacy analysis for further consideration of restricted emergency use approval for the vaccine.
The SEC recommendations on Covishield and in regard to Covaxin have been forwarded to the Drugs Controller General of India (DCGI) for final approval.
The SEC, which earlier had sought additional safety, immunogenicity and efficacy data from the Serum Institute of India (SII) and Bharat Biotech, deliberated on their applications seeking emergency use authorisation (EUA) for their shots on Wednesday, and met again on Friday to review the matter.
"After detailed deliberation, the committee recommended for grant of permission for restricted emergency use of the vaccine (Oxford COVID-19) subject to various regulatory provisions," a source said.
While granting the restricted emergency use approval for the Oxford COVID-19 vaccine, the panel imposed certain regulatory provisions, including that the shot is indicated for active immunisation in individuals of 18 years or more to prevent the disease and that it should be administered intramuscularly in two doses at an interval of four to six weeks.
Further, SII should submit safety, efficacy and immunogenicity data from the ongoing clinical trials in the country and across the globe for review at the earliest.
Pune-based SII should also submit safety data including the data on adverse events following immunisation (AEFI) and adverse event of special interest (AESI) with due analysis every 15 days for the first two months and monthly thereafter till the completion of the ongoing clinical trial in the country, according to the recommendations.
SII is the world's largest vaccine manufacturer and has tied up with AstraZeneca to manufacture Covishield.
According to sources, the firm presented the details of the conditions and restrictions under which AstraZeneca was granted emergency use authorisation in the United Kingdom and the revised fact sheet and information in the Indian context as required by the committee.
The UK's Medicines and Healthcare Products Regulatory Agency (MHRA) approval dated December 30 along with its conditions/restrictions was also reviewed by the committee on Friday. It noted that the safety and immunogenicity data presented by the firm from the Indian study is comparable with that of the overseas clinical trial data.
While considering Bharat Biotech's application, the SEC noted that the ongoing clinical trial is a large one with 25,800 subjects of which 22,000 have been enrolled, including subjects with comorbid conditions, which has demonstrated safety till date but efficacy is yet to be demonstrated.
"After detailed deliberation, the committee recommended that the firm should try to expedite the recruitment and may perform interim efficacy analysis for further consideration of restricted emergency use approval," an official source said.
The firm had presented safety and immunogenicity data, including serious adverse event data from the phase 1 and 2 clinical trial along with the data from the ongoing phase 3 clinical trial in the country. Pfizer did not turn up for the deliberation, sources said.
The UK's MHRA on Wednesday had approved the COVID-19 vaccine developed by scientists at Oxford University and produced by AstraZeneca for human use.
'In terms of safety, Covishield was well tolerated with respect to solicited adverse events... majority of solicited reactions were mild in severity and resolved without any sequelae. Therefore, Covishield is safe and can be used effectively for prevention of COVID-19 in the targeted population. Thus, the benefit to risk ratio strongly supports the widespread use of Covishield,' the EUA application signed by Prakash Kumar Singh, Additional Director, Government and Regulatory Affairs at Serum Institute of India (SII), had stated.
'In line with our philosophy, we assure you that for COVID-19 vaccines also, we are committed to make our country 'Atmanirbhar' and fulfil our Prime Minister's clarion call of 'vocal for local' and 'making in India' for the world,' Singh had stated in the application.
SII had applied to the Drugs Controller General of India for EUA for Oxford COVID-19 vaccine on December 6, while Hyderabad-based Bharat Biotech had sought the nod for its indigenously developed Covaxin on December 7.
Pfizer had applied for regulatory approval for its vaccine on December 4.