After reports claimed that filings in patent offices in India, the US, and Europe did not mention ICMR or its scientists, but only BBIL and its scientists, the Hyderabad-based biotechnology (biotech) company clarified that in the “rush” to develop vaccines and file appropriate patents, BBIL had missed adding ICMR’s name in the original filings.
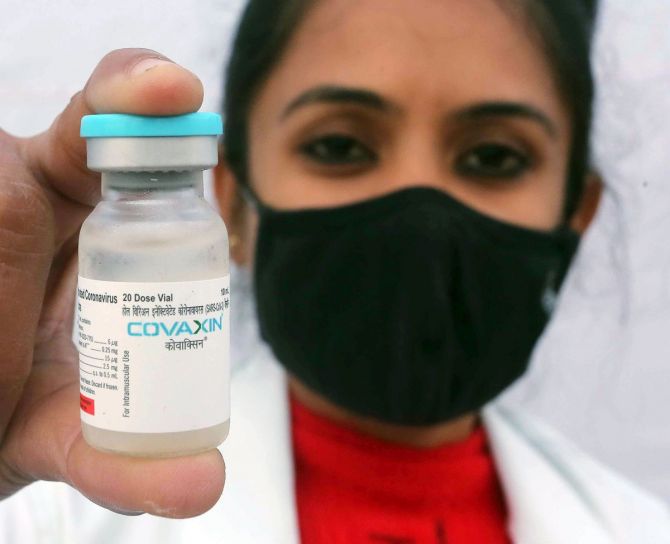
Experts in the scientific community feel that giving credit to someone for their contribution to a patent filing is indeed desirable. Bharat Biotech International (BBIL) has initiated the process of including the Indian Council of Medical Research (ICMR) as a co-owner in the patent application for the Covid vaccine Covaxin.
A senior virologist who has worked closely with the ICMR on several of their committees told Business Standard, “It is desirable to give credit to someone.” However, he felt the conflict of interest that arises from a regulatory agency making profits from their recommendations is not desirable.
BBIL said during the weekend that it has initiated a process to include ICMR as co-owner in the patent application for Covaxin.
The company claimed that it was an “inadvertent” mistake to not include ICMR in patent filings.
After reports claimed that filings in patent offices in India, the US, and Europe did not mention ICMR or its scientists, but only BBIL and its scientists, the Hyderabad-based biotechnology (biotech) company clarified late at night that in the “rush” to develop vaccines and file appropriate patents, BBIL had missed adding ICMR’s name in the original filings.
“Bharat Biotech was working on developing the Covid vaccine on priority to ensure product availability at the earliest. The Covid vaccine development of BBIL was faced with multiple challenges and all organisations were in a rush to develop vaccines and file the appropriate patents, prior to any other entity or prior to any data being published in journals,” the firm said. It added that the vaccine application was filed in the above circumstances and the BBIL-ICMR agreement copy (being a confidential document) was not accessible.
“Hence, ICMR was not included in the original application. Although this was purely unintentional, such mistakes are not uncommon for the patent office; therefore, patent law provides provisions to rectify such mistakes,” the company said.
While it is not clear why ICMR was not included in the original patent filings, researchers felt that including other parties in patent filings is not that rare in the scientific world.
“Patent applications are primarily to protect intellectual property (IP). Researchers move to protect their IP in a rush, and that is not uncommon. Also, when there are significant commercial implications of the decision, all parties try to protect their interests,” said a senior researcher working in the field of biotech.
BBIL said on Saturday that it has “great respect” for ICMR and is thankful to ICMR for their continuous support on various projects. Therefore, as soon as this inadvertent mistake was noticed, BBIL has already started the process to rectify it by including ICMR as co-owner of the patent applications for the Covid vaccine, it claimed.
“Necessary legal documents are being prepared for it and BBIL will file those documents in the patent office as soon as those are ready and signed. These actions are in accordance with the memorandum of understanding (MoU) signed between ICMR-National Institute of Virology Pune and BBIL for joint development of the Covid vaccine in April 2020,” the company said.
In a Rajya Sabha (RS) response, then Minister of State for the Ministry of Health and Family Welfare Bharti Pravin Pawar had said in July 2021 that the MoU between ICMR and BBIL included terms like collaboration for the development of Covid inactivated whole-cell vaccine and that ICMR would provide a well-characterised virus strain for vaccine development.
ICMR would get a royalty payment of 5 per cent on net sales to be paid on a half-yearly basis, the minister had said. It was also agreed that the vaccine would come in the joint name of ICMR and BBIL, and ICMR’s logo would be there on the product.
Further, in a February 2022 RS response, Pawar had informed that ICMR had received a royalty payment of Rs 171.74 crore until January 2022 from BBIL from sales of Covaxin. “ICMR has spent around Rs 35 crore on the research and development of Covaxin,” the minister had said.











 © 2025
© 2025