India is expecting 156 million doses of the vaccine from August to September, reports Ruchika Chitravanshi.
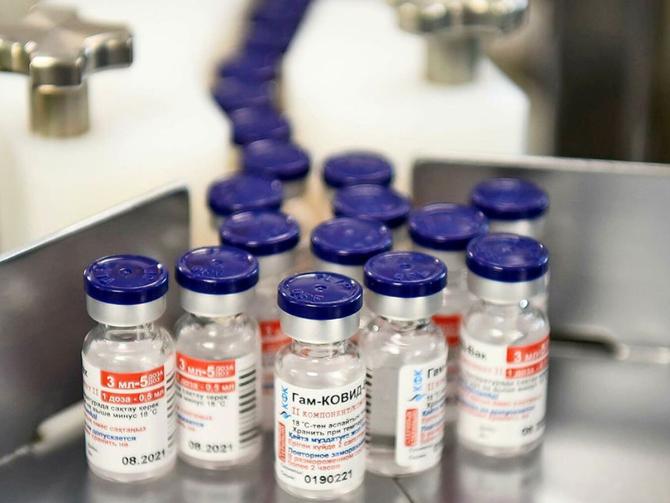
Sputnik V, the vaccine developed by Russia’s Gamaleya National Center Covid, will be available in India from next week, the Union ministry of health and family welfare announced on Thursday.
V K Paul, member (health) of the NITI Aayog, said on Thursday while addressing a press briefing: “I am very happy to say the limited supply that has come from Russia will start selling in India and more supplies will come in,” said Paul.
India is expecting 156 million doses of the vaccine from August to September.
Sputnik V, a vaccine with 91.6 per cent efficacy -- the highest among Covid vaccines available in India -- requires a temperature range of -18°C to -22°C to remain stable.
Sputnik V will be imported in the frozen form from Russia this quarter.
DRL is responsible for ensuring that the vaccine remains stable and sanctity of the cold chain is maintained -- from the manufacturing site in Russia to its cold chain point and eventually to all parts of India.
As of now, DRL’s contract with the Russian Direct Investment Fund is for 250 million doses for India.
Deepak Sapra, chief executive officer (API and pharmaceutical services), DRL, had said last month that the company had lined up a solution of compact boxes, which would help transport vaccines to various parts of India easily through a combination of air and road routes.
Meanwhile, stability data for the Sputnik V variant that will remain stable at 2-8°C is being generated.
The government is also expecting Zydus Cadilla’s DNA vaccine, towards the end of its phase three trial, to apply for licence soon.
The single drop, single dose nasal vaccine is also expected to add to the supply of vaccines in India by almost 100 million, Paul said.
The NITI Aayog recently said that India would examine the claim whether a single dose of Sputnik Light can provide protection from the coronavirus infection.
Paul had noted that if the claim of the vaccine developer was true, it could help double the speed of vaccination in India.
Covaxin technology transfer
The health ministry has clarified that there is no delay in licensing of Covaxin or approval for technology transfer for its manufacturing.
Paul said, “We have given open invitation to companies to make this vaccine but there is a constraint of technological platform.”
Covaxin uses a live virus, which is inactivated.
This requires a highly sophisticated standard of lab -- BSL3 -- which is not available with most pharma companies.
Two public sector units, Indian Immunologicals and BIBCOL have entered into a technology transfer agreement with Bharat Biotech along with Haffkine Institute, a state government undertaking.
With inputs from agencies











 © 2025
© 2025