Terming World Health Organisation's decision to resume testing of hydroxychloroquine as a potential treatment for COVID-19 in its global clinical trial "a step in the right direction", experts said any "positive outcome" of the exercise will be in the larger interest of the people globally.
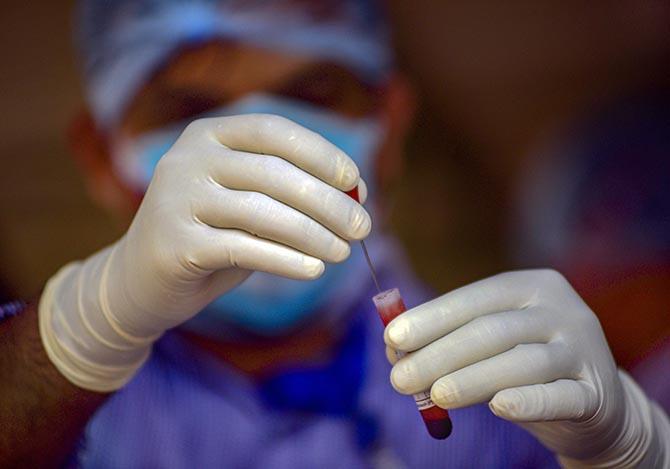
The global heath body had earlier suspended the hydroxychloroquine arm of the clinical trials of experimental COVID-19 drugs over safety concerns.
However on Wednesday, it recommended the trial to be continued following a review of safety data.
"On the basis of the available mortality data... the members of the Solidarity Data Safety Monitoring Committee unanimously agreed that there are no cogent reasons to recommend modifications of the protocol of the trail and advised that the trial should be continued as planned," the Executive Group of the Steering Committee of the Solidarity Trial wrote to all National Principal Investigators of the Solidarity Trial.
Welcoming WHO's decision, ICMR Director General Dr Balram Bhargava said, "ICMR and India have been firm on recommendations about the drug based on biological plausibility, in vitro data and case controlled studies.
"It is a time tested drug in use for decades. Any positive outcome from the clinical exercise will be in the larger interest of the people globally."
He had earlier said that no major side-effects of the anti-malarial drug hydroxychloroquine has been found in studies in India and its use can be continued as prophylaxis for COVID-19 under strict medical supervision.
AIIMS Director Dr Randeep Guleria said WHO's decision about resuming the HCQ arm of the clinical trial was "a step in the right direction towards larger public interest".
"Data from India both from AIIMS and ICMR shows a good safety profile. We did not find that this drug was causing significant cardiac toxicity and therefore it is good that WHO reviewed their data and reintroduced the drug in the trial.
"This is a drug which is less expensive, easily available and has been used widely for a long time with good safety data. It will be good if the medicine turns out be beneficial in some way in COVID-19 treatment," he said.
Dr Sheela Godbole, the National Coordinator of the WHO-India Solidarity Trial and Head of the Division of Epidemiology, ICMR-National AIDS Research Institute said the hydroxychloroquine arm of the Solidarity Trial alone was paused as the Solidarity Data Safety Monitoring Committee reviewed the data.
"Yesterday, the report of this committee was received and they advised that the trial should be continued as planned. Based on this, the Executive Group has restarted the hydroxychloroquine arm.
The world needs strong data from well-conducted randomised controlled clinical trials on the drug for treatment of COVID-19. We are glad that we can begin the hydroxychloroquine arm again," Dr Godbole said.
Besides hydroxychloroquine, three more treatment protocols -- remdesivir, comibnation of lopinavir and ritonavir, and lopinavir and ritonavir with Interferon beta-1a -- are being evaluated during the clinical trials at selected hospitals in the world.
The Indian Council of Medical Research (ICMR) in its revised advisory on May 22 recommended use of the drug as a preventive medication for COVID-19 for asymptomatic healthcare workers in non-COVID hospitals and frontline staff on surveillance duty in containment zones and paramilitary/police personnel involved in coronavirus infection related activities.
The drug is also recommended for all asymptomatic healthcare workers involved in containment and treatment of COVID-19 and household contacts of laboratory confirmed cases.
The Union health ministry on March 31 had also recommended use of hydroxychloroquine in combination with azithromycin on COVID-19 patients who are in severe condition requiring ICU management.
Lancet issues 'expression of concern' after scientists question validity of HCQ study
The Lancet journal has issued a statement of concern after over 100 scientists from across the world flagged discrepancies in its recent study linking the malaria drug hydroxychloroquine with increased death risk during COVID-19 treatment.
"We are issuing an Expression of Concern to alert readers to the fact that serious scientific questions have been brought to our attention. We will update this notice as soon as we have further information," the editors of the journal said.
The statement has come after more than 100 scientists from across the globe wrote an open letter to the editor of The Lancet, Richard Horton, questioning the validity of the study which assessed the safety and effectiveness of hydroxychloroquine (HCQ) in COVID-19 treatment.
The research, which was published on May 22, was an observational study of 96,032 hospitalised COVID-19 patients from six continents that reported substantially increased deaths, and incidences of heartbeat rhythm changes associated with the use of the drugs HCQ and closely related chloroquine.
Based on the study, the scientists had concluded that the drugs are "associated with decrease in-hospital survival and an increased frequency of ventricular arrhythmias when used for treatment of COVID-19."
Soon after the study was published, the World Health Organisation (WHO) paused recruitment of patients to the HCQ arm in their SOLIDARITY clinical trial, which they resumed on Wednesday after the scientists questioned the study.
"If we don't have a double blind randomised controlled trial (RCT), where the neither the doctors nor the patients know what drug they are on, conclusions are always subject to bias, and The Lancet study was not and RCT," explained Ram Vishwakarma, Director of CSIR-Indian Institute of Integrative Medicine (CSIR-IIIM) in Jammu.
Vishwakarma told PTI that the ethical process in publishing scientific studies is to disclose the database being used in any study, which he said was not followed in The Lancet research.
"Important scientific questions have been raised about data reported in the paper by Mandeep Mehra et al -- Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis -- published in The Lancet on May 22, 2020," said the editors of The Lancet in their statement expressing concern.
The research was based on a database from a company based in Illinois, US called Surgisphere Corporation, which according to the study contains COVID-19 patient data from hundreds of hospitals around the world.
From this database, the study assessed data from 96,032 patients admitted to 671 hospitals across six continents by April 14, of whom, 10,698 had died in hospital by April 21, according to the research.
However, in the open letter, the researchers flagged several points of concern about the validity of this data, and the kind of analysis done in the study with it.
Among the major issues cited in the Lancet study by the scientists, are concerns that there was no mention of the countries or hospitals that contributed to the data source and no acknowledgments of their contributions.
The data reported in the study from Australia, for instance, the open letter said, was not compatible with government reports from the country.
Data from Africa, for instance, indicated that nearly 25 per cent of all COVID-19 cases and 40 per cent of all deaths in the continent occurred in "Surgisphere-associated hospitals" which had patient monitoring facilities that could detect and record "nonsustained or sustained ventricular tachycardia or ventricular fibrillation".
"Both the numbers of cases and deaths, and the details provided, seem unlikely," the scientists flagged in the open letter.
Scientists also noted that the mean daily doses of HCQ reported in the Lancet study are 100 milligrames (mg) higher than US Food and Drug Administration recommendations, while as much as 66 per cent of the data are noted from North American hospitals.
In the expression of concern raised by The Lancet on Wednesday, they said "an independent audit of the provenance and validity of the data has been commissioned by the authors not affiliated with Surgisphere and is ongoing."
"The expression of concern means that no scientist or doctor should be biased from the study," Vishwakarma explained.
"But that doesn't mean the study is wrong. Now the author's replies need to come and if they have all the evidence supported, then the study will stand, otherwise this paper will have to be retracted," he said.









 © 2025
© 2025