Hetero has priced the injectable drug at Rs 5,400 per 100 mg vial. With more drugmakers in line to launch the drug soon, the prices may see a further erosion.
Sohini Das reports.
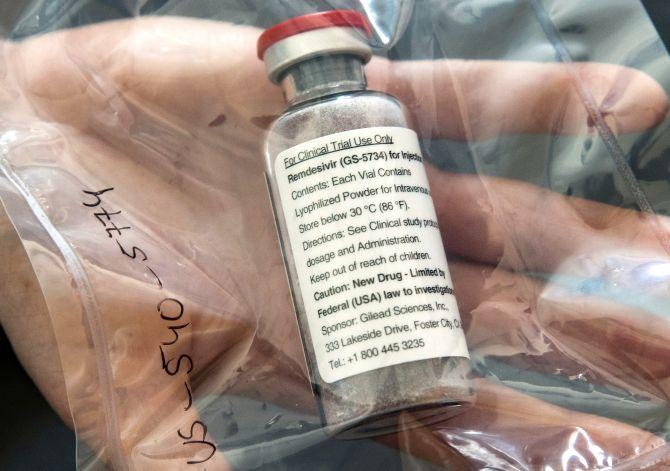
The pricing war for antiviral drug Remdesivir has begun in the domestic pharmaceutical market. Two Indian drug majors -- Cipla and Hetero Healthcare -- have come out with the final pricing of their products.
Hetero has priced the injectable drug at Rs 5,400 per 100 mg vial. Cipla said it would price its generic version of Gilead Sciences’ antiviral drug for use in Covid-19 patients at less than Rs 5,000 per vial. Each patient will need six vials for the course of treatment.
Commenting on the drug’s pricing, a spokesperson for Cipla said, “In line with our overall philosophy of driving access and affordability, the drug will be priced at less than Rs 5,000 per vial for 100 mg injection -- among the lowest pricing for Remdesivir globally.” Cipla will be selling it under the brand Cipremi. Hetero’s brand is Covifor. Sources revealed that Cipla may price the drug around Rs 4,000 per dose.
More drugmakers, including Jubilant Pharma, Mylan, Cadila Healthcare, and Dr Reddy’s Laboratories, are in line to launch this drug soon. The prices may see further erosion then.
A senior government official said it is in wait-and-watch mode and has not decided to take any suo motu action on the pricing of the drug. Cipla had signed an agreement to contract the manufacture of Remdesivir with BDR Pharmaceuticals International recently. BDR had transferred the formulation technology and other methods of development to Sovereign Pharma.
Hetero and Cipla received approval from the country’s drug regulator to launch the drug this weekend. Hetero said it is set to deliver the first set of 20,000 vials in two equal lots of 10,000 each, one of which will be immediately despatched to Hyderabad, Delhi, Gujarat, Tamil Nadu, Mumbai and parts of Maharashtra.
The other lot will be supplied to Kolkata, Indore, Bhopal, Lucknow, Patna, Bhubaneswar, Ranchi, Vijayawada, Cochin, Trivandrum, and Goa within a week’s time to meet emergency requirements.
M Srinivasa Reddy, MD, Hetero Healthcare, said: “Through Covifor, we hope to reduce the treatment time of a patient in a hospital, thereby reducing the increasing pressure on the medical infrastructure, overburdened currently due to accelerating Covid-19 infection rates. We are working closely with the government and the medical community to make Covifor quickly accessible to both public and private health care settings across the country.”
Hetero had earlier said it is diverting some of its export stocks to the Indian market to meet local demand. Hetero has even started a toll-free helpline to support doctors and hospitals interested in procuring the drug.
The drug is available in a 100-mg injectable vial. It needs to be administered intravenously in a hospital (critical care setting) under supervision of a registered medical practitioner.
Meanwhile, Glenmark is also working towards making Favipiravir available soon across the country. The company has already made it available in Delhi, Haryana, and Himachal -- these are areas near the site of manufacturing in Baddi.
Within a week, the drug will be available across the country.
Sources claim Cipla’s price would lower by 30 per cent, compared to Glenmark’s. The company did not verify this.
A source close to the development said while mild patients are to be given Favipiravir, if someone does not show symptoms of fever, clinicians may choose not to do so. Each tablet costs Rs 103 and Glenmark is selling it as a pack of 34 tablets. “Around 1,000-2000 patients per day would be prescribed Favipiravir,” added a source.
Some companies have geared up to launch the drug and more brands will hit the market in a month’s time, bringing prices down.











 © 2025
© 2025